Abstract
The treatment of BL/L has evolved on the principles of short-course, intensive, non-cross resistant, alternating chemotherapy. These intensive high-dose methotrexate-based treatment regimens (CODOX-M/IVAC, Hyper-CVAD, BFM, LMB) have led to high response rates and cures in a significant proportion of children and young adults. The addition of rituximab has led to overall survival benefit. There is limited data that dose adjusted EPOCH-R provides a low intensity treatment option for patients who may not be able to tolerate high-dose methotrexate-based regimens. Efficacy of these regimens in real-world, more so in an LMIC setting need to be evaluated given the delays in seeking care, higher risk of treatment-related complications including infections and antecedent treatment interruptions.
This retrospective multicentric study collected the data from eight-member centers of Hematology Cancer Consortium (www.hemecancer.org) using an electronic database, to analyze the clinical characteristics, treatment patterns, outcomes and prognostic factors in adolescent and adult newly diagnosed Burkitt lymphoma and leukemia diagnosed between 2012-2019 (including HIV positive). Patients who had received more than 2 weeks of chemotherapy or steroids prior to presentation were excluded. The data included demographic details, performance status (ECOG), HIV serology, bone marrow and CNS involvement, stage, treatment type, use of rituximab, response to treatment, and treatment-related mortality as assessed by the investigator of the respective centre. The primary objective was to evaluate event-free survival at 2 years. Secondary objectives were to evaluate the overall survival, impact of the treatment protocol, use of rituximab, stage, age, performance status, CNS involvement, and HIV positive status on the overall and event-free survival.
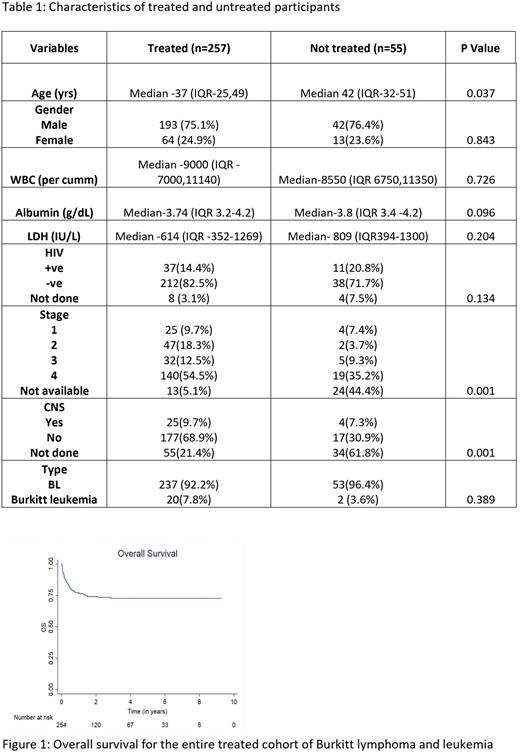
A total of 312 patients were included in this study. Out of these 257 patients received treatment and were analyzed for the outcome and prognostic factors. The treated and untreated cohorts differed in age [ median age 37 years (range-25-49 years) versus 42 years (range-32-51 years); with the untreated cohort being older. Table 1 provides the baseline characteristics of the patients who received treatment. The HIV positive patients (in treated cohort) had higher LDH [median - 967.5 (range, 509- 2355) vs 589 (311-1141), p=0.003). A total of 100 (42%) patients received intensive and high-dose methotrexate-based chemotherapy, 81 (34%) patients received dose-adjusted EPOCH-based treatment whereas 56 (24%) received other low-intensity or palliative chemotherapy. Patients with HIV were largely treated with a dose-adjusted EPOCH-based regimen (25/32 patients). Patients who received a high-dose methotrexate-based regimen were younger than those who received dose-adjusted EPOCH and other lower intensity regimens (Mean age 29 years versus 42.4 and 43.9 years)
At the median follow-up of 36.5 months, the 2-year EFS was 61% and 2-year OS was 73%. There was no significant difference in outcomes in HIV-positive and negative patients. On univariate analysis lack of use of rituximab and use of protocols other than high-dose methotrexate and dose-adjusted EPOCH negatively affected EFS and OS. Age had an adverse impact on the OS but not EFS whereas stage 2 or above had a negative impact on EFS.
On multivariate analysis use of regimens other than high-dose methotrexate-based and dose-adjusted EPOCH was a negative prognostic factor for both EFS and OS. The HR favored high dose methotrexate and dose-adjusted EPOCH-based regimen for both EFS and OS; HR- 0.42 (95% CI,0.22,0.77) and 0.27(95% CI,0.13-0.59), respectively. Use of rituximab was associated with better OS HR-0.44(95% CI,0.21-0.93) and a trend toward better EFS - HR-0.58 (95%CI, 0.31-1.07). The treatment-related mortality was 11.2% (29/257) and high serum LDH was associated with higher mortality (p=0.003).
Treatment of adolescent and adult Burkitt lymphoma and leukemia with intensive regimens (high-dose methotrexate-based) and dose-adjusted EPOCH regimen resulted in similar survival. Dose-adjusted EPOCH-based regimen was preferred in older patients. The use of rituximab in these patients adds to the overall survival benefit.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal